The pREBOA-PRO™ catheter designed for True Partial REBOA™ gives physicians time to treat patients bleeding or at risk of bleeding to death
SAN ANTONIO, Dec. 5, 2023 /PRNewswire-PRWeb/ -- Prytime Medical Devices Inc, The REBOA Company™, the leading innovator and global provider of lifesaving REBOA products for minimally invasive, endovascular hemorrhage control, announced reaching the milestone of 500 patient uses with its lifesaving pREBOA-PRO™ catheter.
The 500th patient was brought into Northeast Georgia Medical Center in stable condition, but with life-threatening injuries, when their blood pressure began to crash.
"We inserted the pREBOA-PRO™ catheter and used partial REBOA for 52 minutes. It gave us time to stabilize and treat the patient's wounds," said Michael Cormican, MD, a trauma surgeon with Northeast Georgia Physicians Group. "This device has helped provide our trauma team with the tools they need for patients with penetrating injuries."
"We congratulate Dr. Michael Cormican for this milestone," said David Spencer, CEO of Prytime Medical Devices, Inc. "Northeast Georgia Medical Center (NGMC) is one of sixteen Level 1 and 2 trauma centers across North America who are part of a limited release of the pREBOA-PRO™ catheter. These sixteen hospitals are Centers of Excellence in which all aspects of the partial REBOA catheter and procedure are evaluated and studied to improve clinical outcomes. NGMC demonstrates that regional, community trauma centers can have high performing REBOA programs when supported by well-trained teams and institutional protocols."
Too Many Patients Are Still Bleeding to Death
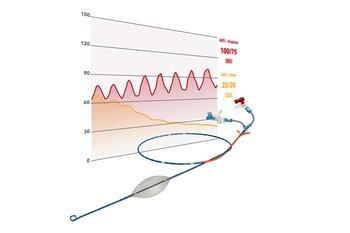
Time is the enemy of trauma surgeons. A landmark study in 2018 (Holcomb et al, 2018) showed that it can take bleeding patients over 2 hours to get definitive care after they finally arrive at the hospital. These hemodynamically unstable patients are typically so sick that their need for REBOA exceeds the standard 30 minutes usage in aortic Zone 1. Partial REBOA, or partial occlusion of the aorta, allows the viscera to be sufficiently perfused during the REBOA procedure.
"When we launched pREBOA-PRO™ in 2021, we assumed that partial REBOA using pREBOA-PRO™ would have an enhanced value proposition from complete REBOA. But, we did not anticipate how truly different the value and the clinical uses would be once you break the 30-minute Zone 1 barrier," Spencer continued. "After two and a half years working closely with some of the top trauma centers in North America, we can now see how impactful and measurable those differences are."
Clinical Experience So Far with the pREBOA-PRO™ Catheter
A recent summary of published literature highlights three key clinical benefits for the pREBOA-PRO™ catheter:
- Buying time for extended safe occlusion times beyond 30 minutes (Footnotes 2, 3, 4, 5, 6, 7)
- Expanding treatment options including more CT scans and less blood use (Footnotes 7, 8, 9)
- Reducing serious complications due to Acute Kidney Injury (AKI) (Footnotes 10, 11, 12, 13, 14)
"pREBOA-PRO™ gives physicians much-needed time – time to resuscitate, time to stabilize, time to evaluate, and simply, time to treat the patient," continued Spencer. "The physicians at the Centers of Excellence continue to invest in additional research to broaden our understanding of partial REBOA using the pREBOA-PRO™ catheter."
In March 2023, Prytime Medical announced the launch of the Partial REBOA Outcomes Multicenter ProspecTive (PROMPT) Study, the first large-scale multicenter prospective study for Partial REBOA. There are four key endpoints for the study: 1. Time of occlusion 2. Ischemic markers 3. Tolerance to reperfusion 4. Blood product use. Currently, there are five sites enrolling with more to be announced.
"We are committed to replacing anecdote with data to advance and accelerate the science of REBOA which will improve patient outcomes and save lives," concluded Spencer.
About Prytime Medical Devices, Inc.
Prytime Medical™ Devices, Inc. is an innovative medical device company that designs, develops, and commercializes minimally invasive solutions for hemorrhage control. Our latest innovations, the industry leading ER-REBOA PLUS™, and the innovative pREBOA-PRO™ partial REBOA catheter, enable torso hemorrhage control with more controlled resuscitation in a much wider range of clinical scenarios. pREBOA-PRO™ is the first and only FDA-cleared REBOA catheter designed specifically for True Partial REBOA™. More information can be found at http://www.PrytimeMedical.com
About Northeast Georgia Medical Center
Since 1951, Northeast Georgia Medical Center (NGMC) has been on a mission of improving the health of our community in all we do. With hospitals located in Gainesville, Braselton, Winder, Dahlonega and Demorest, the five NGMC campuses have a total of more than 850 beds and more than 1,300 medical staff members representing more than 60 specialties. NGMC is part of Northeast Georgia Health System, a non-profit that cares for more than one million people across more than 19 counties. Learn more at http://www.nghs.com
Footnotes
- Holcomb et al , Transport Time and Preoperating Room Hemostatic Interventions Are Important: Improving Outcomes After Severe Truncal Injury, Crit Care Med 46(3) (2018) 447-453.
- Prytime Medical Centers' of Excellence. Data on file.
- Polcz et al., (2022) J Surg Res. Based on preclinical data. Clinical results in humans are unknown.
- Edwards et al., (2022) J Am Coll Surg. Clinical results in humans are unknown.
- Necsoiu et al., (2021) Shock. Based on preclinical data. Clinical results in humans are unknown.
- Kemp et al., (2021) J Trauma Acute Care Surg. Based on preclinical data. Clinical results in humans are unknown.
- Ho et al., (2023) J Trauma Acute Care Surg. Clinical results in humans are unknown.
- Nguyen et al., (2023), Maximizing patient care: Partial REBOA enables increased use of CT imaging and endovascular hemorrhage control, AAST poster
- Nguyen et el., (2024), pREBOA vs ER-REBOA Impact on Blood Utilization and Resuscitation Requirements: A Pilot Analysis, EAST presentation
- Russo et al., (2020) J Trauma Acute Care Surg.
- Gomez et al., (2023) J Trauma Acute Care Surg.
- Ronaldi et al., (2021) Shock. Based on preclinical data. Clinical results in humans are unknown.
- Madurska et al., (2021) Eur J Trauma Emerg Surg.
- Hunt et al., (2023) The American Surgeon
Media Contact
Jeffrey Jung, Prytime Medical Devices, Inc., 210-544-1752, [email protected], https://prytimemedical.com/
SOURCE Prytime Medical Devices, Inc.




Share this article