In its 12th annual Healthcare Fraud & Abuse Review, Bass, Berry & Sims provides analysis of healthcare fraud enforcement actions and False Claims Act developments probing SCOTUS cases, opioid enforcement and other areas.
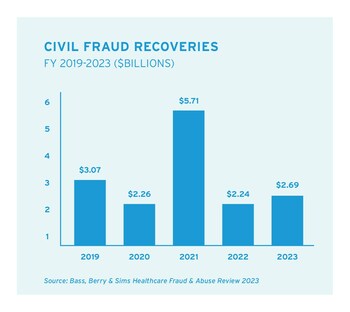
NASHVILLE, Tenn., Feb. 28, 2024 /PRNewswire-PRWeb/ -- The U.S. Department of Justice (DOJ) reported civil fraud recoveries of nearly $2.7 billion in fiscal year (FY) 2023, with two-thirds of those recoveries in the healthcare industry, according to the 12th annual Healthcare Fraud & Abuse Review published today by law firm Bass, Berry & Sims.
Qui tam lawsuits filed by whistleblowers under the False Claims Act continue to be a driving force behind the government's civil healthcare fraud enforcement efforts. The government recovered nearly $1.9 billion in settlements and judgments in matters involving the healthcare industry in FY2023, which amounted to 68% of DOJ's total civil fraud recoveries. Whistleblowers filed more than 700 qui tam lawsuits in FY2023, with over 6,500 hundred such lawsuits filed in the last decade.
Download the Healthcare Fraud & Abuse Review 2023.
"The government and whistleblowers remain keenly focused on alleged violations of the Anti-Kickback Statute and alleged violations of the False Claims Act involving managed care," said Brian D. Roark, co-chair of the Bass, Berry & Sims Healthcare Fraud & Abuse Task Force. "Individuals and entities in the healthcare industry should expect continued focus in these areas in the coming year. Other hot-button issues likely will include the intersection of the False Claims Act with cybersecurity issues and the Controlled Substances Act, among others."
Supreme Court Weighs in on Key False Claims Act Issues
The Review provides a comprehensive update on major trends in False Claims Act enforcement, including detailed analysis of important federal court decisions. Last year, the Supreme Court issued important opinions concerning the False Claims Act's scienter standard (U.S. ex rel. Schutte v. SuperValu Inc.) and the authority of the government to dismiss a False Claims Act lawsuit over the objection of the whistleblower who filed the lawsuit (U.S. ex rel. Polansky v. Executive Health Resources).
"The Supreme Court infrequently considers False Claims Act issues, but when it does, its rulings can alter the enforcement landscape for healthcare companies and government contractors," said Matthew M. Curley, co-chair of the Bass, Berry & Sims Healthcare Fraud & Abuse Task Force and editor of the Healthcare Fraud & Abuse Review 2023. "The Court's rulings in Schutte and Polansky settled important questions concerning scienter and the government's False Claims Act dismissal authority, respectively, and the impact of those decisions will reverberate in False Claims Act investigations and litigation and moving forward."
Controlled Substances Act Enforcement
The opioid crisis has prompted changes in the way the federal government seeks to enforce the Controlled Substances Act, which has been in effect for more than 50 years. For most of the Controlled Substances Act's history, the government has focused on individuals who diverted controlled substances from proper medical uses to illicit uses, such as personal consumption or resale. The Review details how the government recently has changed its enforcement focus to go beyond individuals responsible for diversion to enforce the Controlled Substances Act against healthcare organizations involved at any step in the controlled substances supply chain, including manufacturers, distributors, pharmacies and practitioners, resulting in multi-million-dollar settlements.
"When controlled substance diversion is discovered at a healthcare organization, the federal government no longer takes the view that it should simply focus on the potential prosecution of a diverting employee," said Lisa S. Rivera, a member of the Healthcare Fraud & Abuse Task Force and a co-chair of the firm's Controlled Substances Enforcement & Diversion Practice. "Instead, the government is demanding answers about how the diversion could have occurred, and when it should have been discovered, focusing on the organization's record keeping, security and reporting practices. Discovery of compliance failures no longer depends on a diversion event, as regulators are now focusing on regular pharmacy audits in pursuing administrative resolution or other means of enforcement."
Providers should expect to see Controlled Substances Act compliance and the False Claims Act continue to intersect in enforcement actions. DOJ sent a strong message to that effect when it intervened in False Claims Act litigation alleging that a national pharmacy chain falsely certified to federal healthcare programs that controlled substance prescriptions it filled were valid, which is covered in the Review.
Compliance guidance updates from DOJ, HHS-OIG
The Review provides an overview of two significant compliance guidance publications in 2023. The DOJ's Criminal Division updated its guidance on how it evaluates corporate compliance programs, while the Office of Inspector General of the U.S. Department of Health and Human Services (HHS-OIG) released its own General Compliance Program Guidance. In 2024, HHS-OIG will begin publishing industry-specific guidance for different sectors of the healthcare industry.
"HHS-OIG's updated guidance marks the first time in decades that HHS-OIG provided broad compliance guidance," said Anna M. Grizzle, a member of the firm's Healthcare Fraud & Abuse Task Force who advises clients on enforcement and compliance-related issues. "While this guidance echoes past positions, HHS-OIG offered new insights into current areas of focus, such as patient quality and safety considerations, new entrants in the healthcare industry, and financial incentives promoting compliance. DOJ's guidance also emphasized the importance of proactively addressing issues through self-disclosures as part of an effective compliance program. Healthcare providers should evaluate their compliance programs to ensure they follow this new guidance." For a more in-depth look, download the firm's HHS-OIG Year in Review 2023 by clicking here.
The Healthcare Fraud & Abuse Review also highlights issues to watch this year and provides a comprehensive review of settlements involving the healthcare industry, grouped by sector:
- Hospitals & Health Systems
- Hospice & Home Health
- Skilled Nursing Facilities & Nursing Homes
- Pharmaceutical & Device
- Pharmacy Services
- Laboratory, Pathology, Radiology & Diagnostics
- Behavioral Health & Substance Abuse Treatment
- Managed Care & Health Plans
- Specialty Care & Other Provider Entities
- Individual Providers
- Controlled Substances Act
Download the Review here; visit the Bass, Berry & Sims Healthcare Fraud & Abuse Resource Center, which features a database of healthcare fraud settlements since 2012; and follow the Inside the False Claims Act blog to stay up to date on FCA matters.
About Bass, Berry & Sims PLC
Bass, Berry & Sims is a national law firm with more than 350 attorneys dedicated to delivering exceptional service to numerous publicly traded companies and Fortune 500 businesses in significant litigation and investigations, complex business transactions, and international regulatory matters. For more than 100 years, our people have served as true partners to clients, working seamlessly across substantive practice disciplines, industries and geographies to deliver highly effective legal advice and innovative, business-focused solutions. For more information, visit www.bassberry.com.
Media Contact
Erin Ihde, Bass, Berry & Sims PLC, 1 6152596736, [email protected], https://www.bassberry.com/
SOURCE Bass, Berry & Sims PLC




Share this article