Company's 2014-2016 market evidence helped secure consumer access to advanced NAD+ precursor technology
MIAMI, Oct. 9, 2025 /PRNewswire-PRWeb/ -- RevGenetics, a pioneer in longevity and anti-aging supplements, today celebrates the U.S. Food and Drug Administration's decision to reverse its prior stance and confirm that Beta-Nicotinamide Mononucleotide (NMN) is lawful for use in dietary supplements. This pivotal ruling, outlined in the FDA's September 29, 2025 response to citizen petitions from the Natural Products Association and Alliance for Natural Health USA, recognizes that NMN was marketed as a dietary supplement in the United States prior to its authorization for investigation as a new drug.
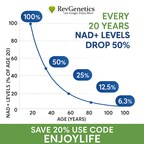
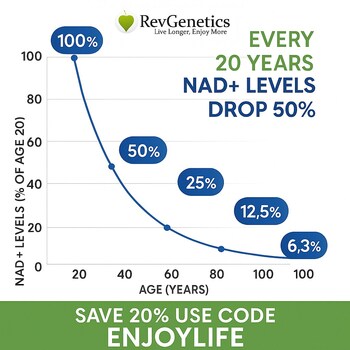
The decision means millions of health-conscious consumers can continue accessing NMN, a supplement that research suggests may help combat age-related NAD+ decline, a key factor in cellular aging, energy metabolism, and overall vitality.
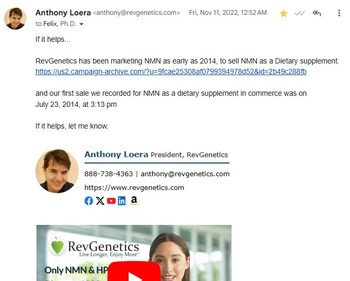
RevGenetics played a key role in this outcome by providing critical evidence of early NMN marketing, including an archived email documenting the company's first recorded sale on July 23, 2014. As one of the earliest adopters and marketers of NMN supplements, RevGenetics has been at the forefront of advocating for NMN's accessibility since 2014, contributing data and insights that helped demonstrate its established presence in the U.S. market.
"RevGenetics has always believed in the safety and efficacy of NMN for supporting healthy aging and cellular energy," said Anthony Loera, President of RevGenetics. "Our pioneering work since 2014, including being the first to market NMN supplements to U.S. consumers, directly contributed to the body of evidence that influenced this FDA reversal. We're proud to have helped pave the way for consumers to access this powerful NAD+ precursor."
The Enteric-Coated Advantage
RevGenetics distinguishes itself as the only provider of enteric-coated NMN with a clean two-ingredient formula. This pharmaceutical-grade delivery system protects NMN from stomach acid degradation and ensures targeted delivery to the small intestine, where the dedicated Slc12a8 transporter facilitates absorption within 30 minutes.
"Standard NMN capsules may face degradation in the acidic stomach environment before reaching the small intestine," Loera explained. "Our enteric coating maintains molecular integrity until reaching the optimal pH of 6 to 7 in the small intestine, where absorption occurs most efficiently."
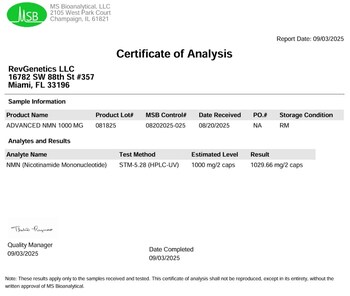
Independent third-party testing confirms each two-capsule serving contains 1029.66 mg of NMN, exceeding the labeled 1000 mg dose. The company has maintained pharmaceutical-grade purity standards and rigorous quality control since its founding in 2007.
Key Questions About NMN Supplements
How does NMN compare to NR (Nicotinamide Riboside)?
While both are NAD+ precursors, recent research from LG Household & Health Care published in Biomedicines (September 2025) reveals significant differences. In transcriptomic analysis of UV-treated skin fibroblasts, NMN triggered robust gene expression changes that protected against aging, while NR showed minimal biological response at equivalent concentrations. The study found NR exhibited only modest increases in cellular NAD+ levels compared to NMN's significant elevation. NMN is one biochemical step closer to NAD+ in the metabolic pathway and enters cells through the Slc12a8 transporter, potentially explaining its superior efficacy.
Why is enteric coating better than sublingual powder?
Sublingual absorption requires rapid dissolution and diffusion across oral mucosa, which is not optimized for NMN's molecular structure. Research shows most sublingual powder is washed away by saliva before meaningful absorption occurs. When 1000mg of powder is placed under the tongue, only 100 to 300mg may absorb through the mucosa before the remainder is swallowed and exposed to stomach acid. In contrast, 1000mg in an enteric capsule delivers nearly the entire dose intact to the small intestine where the Slc12a8 transporter efficiently absorbs it.
How does enteric coating compare to liposomal formulations?
According to Aron Erickson, Vice President of R&D at Niagen Bioscience (formerly ChromaDex), NAD+ precursors "are not stable for long periods of time in water and will degrade rapidly within weeks if maintained in an aqueous environment." Liposomal products with water cores may begin degrading before reaching consumers. Additionally, dried liposomes "readily break open when exposed to processing, like tableting or encapsulation, rendering the liposomes useless for their intended benefit." A 2025 Niagen Bioscience study analyzing 39 NR supplements found that 87 percent failed to meet label claims, with liposomal formulations among the worst performers. RevGenetics' dry, crystalline NMN with enteric coating avoids these stability issues entirely.
Industry Impact and Availability
This FDA decision opens new opportunities for the supplement industry and underscores the importance of early innovators in shaping regulatory outcomes. For a full report and NMN FAQ, visit RevGenetics. RevGenetics Advanced NMN 1000 is available for purchase at RevGenetics.com, with international shipping to many countries worldwide.
For more information, visit RevGenetics.com/products/advanced-nmn-1000-nicotinamide-mononulceotide
About RevGenetics
RevGenetics is a leading provider of science-based longevity supplements, specializing in NAD+ precursors like NMN and Resveratrol. As the first company to market NMN supplements in the United States (2014), RevGenetics continues to innovate with proprietary formulations like enteric-coated NMN, backed by pharmaceutical-grade purity standards and third-party testing. Founded in 2007, the company empowers individuals to live healthier, longer lives through transparent, research-driven products.
Media Contact
Anthony Loera, RevGenetics, 1 305-929-3819, [email protected], https://www.revgenetics.com
SOURCE RevGenetics











Share this article