Voiant announces the publication of a peer-reviewed study in Diagnostics demonstrating a deep learning-based method for automated geographic atrophy (GA) quantification in SD-OCT imaging. The study validates Orion, Voiant's cloud-based reading platform, as a scalable and reproducible solution for multimodal GA and EZ analysis in clinical trials.
WALTHAM, Mass., Nov. 6, 2025 /PRNewswire-PRWeb/ -- Voiant, a leader in clinical trial imaging solutions, is pleased to announce the publication of a new peer-reviewed study in Diagnostics as part of the Special Issue Updates on the Diagnosis and Management of Retinal Diseases—2nd Edition. The article, titled "Deep Learning-Based Segmentation of Geographic Atrophy: A Multi-Center, Multi-Device Validation in a Real-World Clinical Cohort", presents a deep learning-based method for automated segmentation and quantification of geographic atrophy (GA) in spectral-domain optical coherence tomography (OCT) scans.
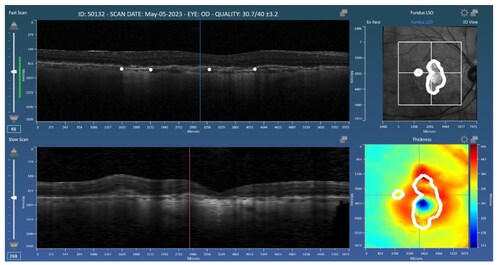
The model was trained and validated on real-world clinical data from two major OCT platforms (Spectralis and Cirrus) among patients with and without concurrent neovascular age-related macular degeneration (nAMD). Results from the Spectralis and Cirrus data sets achieved Dice similarity coefficient (DSC) scores of 0.83 and 0.82, respectively, and correlation coefficients (r2) of 0.91 and 0.88, respectively, with manual expert grading. The study concludes that deep learning offers accurate, reproducible GA assessment across diverse imaging conditions and comorbidities. Read the full open-access paper here for more information.
The research leverages Orion, Voiant's cloud-based reading platform developed by Voxeleron, a Voiant Company. Orion supports flexible reading center workflows with advanced editing tools, native file format compatibility, and automated segmentation for retinal layers, GA, and fluid regions. The purpose-built software enables multimodal analysis tools that are validated, reproducible, and tailored to sponsor needs.
The paper, first authored by Dr. Hasenin Al-khersan of the Retina Consultants of Texas, was co-authored by Voiant Senior Vice Presidents and co-founders of Voxeleron, Dr. Daniel Russakoff and Dr. Jonathan Oakley, along with Voiant's Ophthalmology Scientific Advisory Board members Dr. David Boyer and Dr. Charles Wykoff.
Dr. Charles Wykoff, Director of Research at The Retina Consultants of Texas and Chairman of the Research and Clinical Trials Subcommittee, Retina Consultants of America, said, "There remains a large unmet need to incorporate quantitative assessment of GA longitudinally into real-world clinical practice, and AI-powered analyses are a critical step in this process. Preliminary data with the current algorithm suggests that it performed well using imaging from different devices and most importantly appeared consistent across different phenotypic variants of AMD, including the common clinical scenario of concurrent nAMD and GA in the same eye."
"This work reflects Voiant's commitment to transparency in data and scientific validation," said Dr. Jonathan Oakley, Voiant Senior Vice President of Imaging & Data Sciences, "We're focused on developing tools that are rigorously tested and trusted by the research community. Orion offers a scalable solution for GA quantification, supporting emerging therapies with reliable, reproducible imaging tailored to applicable clinical needs."
Geographic atrophy is an advanced form of age-related macular degeneration (AMD) and a leading cause of vision loss in older adults. Although there is currently no cure, new therapies that can slow disease progression are emerging, making the need for precise and efficient imaging solutions more critical than ever. Voiant is addressing this gap by developing AI-powered tools that streamline GA endpoint analysis, enabling more consistent and objective measurements across clinical trials.
To request a demo of Orion or speak with our team about how Voiant can support your ophthalmic clinical trial imaging needs, visit https://www.voiantclinical.com/contact/
About Voiant
Voiant is the industry leading AI-based clinical trial imaging solution provider with unparalleled scientific and clinical domain expertise, providing biopharmaceutical companies with high-speed delivery of quality clinical endpoint data.
About Voxeleron
Voxeleron is a pioneering company in AI-driven ophthalmic image analysis, offering advanced solutions for applications in medicine, biology, and beyond. Voxeleron's platform provides image analysis software for all ophthalmic imagery, enabling researchers to accelerate clinical research, generate actionable results, and ultimately drive better patient outcomes.
Media Contact
Vivian Nguyen, VOIANT, 1 888-963-3742, [email protected], https://www.voiantclinical.com/
SOURCE VOIANT


Share this article